Background
BAY 94-9027 (damoctocog alfa pegol; Jivi®) is a B-domain-deleted recombinant factor VIII (FVIII), site-specifically PEGylated to extend its half-life compared with standard-half-life FVIII products, including sucrose formulated FVIII (FVIII-FS; Kogenate® FS/Helixate® FS). A previous pharmacokinetic study confirmed longer half-life and lower clearance with a single dose of BAY 94-9027 compared with a single dose of FVIII-FS. The phase 2/3 PROTECT VIII study (NCT01580293) demonstrated the efficacy, safety and utilization of BAY 94-9027 as prophylactic and on-demand therapy for adolescents and adults with severe hemophilia A, including during major and minor surgeries. This post hoc subgroup analysis of PROTECT VIII aimed to assess the efficacy and safety outcomes of patients with hemophilia A who were receiving FVIII-FS as FVIII replacement therapy prior to study enrollment.
Methods
In PROTECT VIII, 134 patients were treated with BAY 94-9027 for the main, 36-week study period. Patients with ≤1 breakthrough bleed during a 10-week run-in period treated with BAY 94-9027 25 IU/kg twice-weekly (2×W) prophylaxis were considered eligible for randomization and were thus randomized to receive BAY 94-9027 45-60 IU/kg every 5 days (E5D) or 60 IU/kg every 7 days (E7D) for the 26-week study period. Patients with >1 bleed, and thus ineligible for randomization (IFR), and those eligible for randomization but enrolled after randomized arms were full (EFR), were assigned to a 30-40 IU/kg 2×W regimen (Figure 1). In total, 114 patients received BAY 94-9027 prophylaxis and 20 patients were treated on-demand. Patients who completed the main study could enter into an optional extension, in which they could continue to receive BAY 94-9027 on any PROTECT VIII study regimen. This present analysis was designed to assess annualized bleeding rates (ABR), adverse events and FVIII utilization of a subset of PROTECT VIII patients who were previously treated with prophylaxis or on-demand FVIII-FS. Pre-study ABRs were based on patient recall of bleeding events during the 12 months prior to study entry.
Results
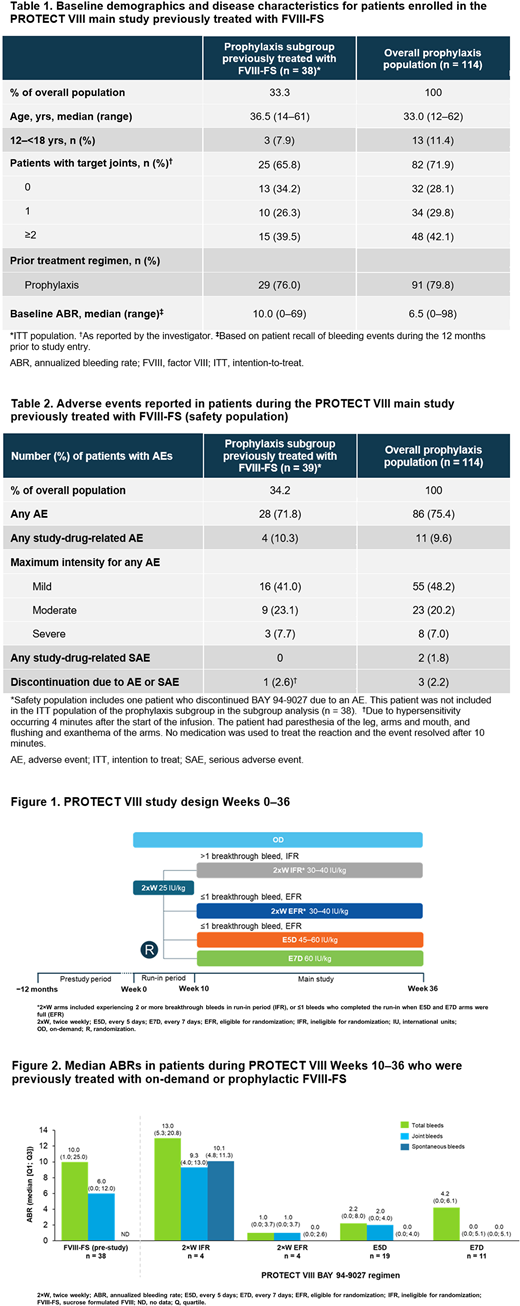
Of the 114 patients who received BAY 94-9027 prophylaxis in the PROTECT VIII main study period, 38 (33.3%) patients were previously treated with FVIII-FS (29 received prior prophylaxis, 9 received prior on-demand treatment) (Table 1). Of these, 8 (21%), 19 (50%) and 11 (29%) patients were treated with 2×W (n = 4 IFR; n = 4 EFR), E5D and E7D BAY 94-9027 regimens in PROTECT VIII, respectively. For patients treated with either prophylactic or on-demand FVIII-FS treatment in the 12-month pre-study period and who were eligible for randomization, median total and joint ABRs decreased during Weeks 10-36 with BAY 94-9027 2×W (EFR), E5D and E7D prophylaxis regimens (Figure 2).
In the 29 patients who switched from FVIII-FS prophylaxis regimens only prior to PROTECT VIII study entry, median (Q1; Q3) total ABRs also decreased from 7.0 (1.0; 14.0) pre-study to 0.0 (0.0; 2.1), 2.1 (0.0; 4.2), and 4.3 (0.0; 5.7) with BAY 94-9027 2×W (EFR), E5D and E7D prophylaxis regimens, respectively. Median joint ABRs also decreased in patients who were previously treated with prophylaxis regimens only with FVIII-FS, from 3.5 (0.0; 8.5) pre-study to 0.0 (0.0; 2.1), 1.1 (0.0; 4.0), and 2.2 (0.0; 5.7) with BAY 94-9027 2×W (EFR), E5D and E7D prophylaxis regimens during the main study period, respectively.
The median dose of BAY 94-9027 administered per infusion in patients who switched from either on-demand or prophylaxis regimens of FVIII-FS was 38.7 IU/kg.
One patient who switched from FVIII-FS discontinued BAY 94-9027 during PROTECT VIII due to an acute, resolved, hypersensitivity episode occurring after the start of the first infusion; no study-drug-related serious adverse events were reported. Overall, of patients who were treated with FVIII-FS in the 12 months prior to PROTECT VIII study enrollment, BAY 94-9027 was well-tolerated and the safety profile was similar to that of the overall prophylaxis patient population in PROTECT VIII (Table2).
Conclusions
Prophylaxis with BAY 94-9027 resulted in lower median total and joint ABRs compared with prior prophylaxis or on-demand treatment with FVIII-FS, resulting in improved efficacy with a similar safety profile.
Reding:Takeda: Consultancy, Honoraria, Speakers Bureau; Sanofi Genzyme: Consultancy, Honoraria, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Speakers Bureau; BioMarin: Research Funding; Bayer: Consultancy, Honoraria, Research Funding, Speakers Bureau. Moulton:Bayer: Current Employment. Soltes Rak:Bayer: Other: Employee of Belcan, contracted with Bayer. Simpson:HEMA Biologics: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Novo Nordisk: Consultancy, Honoraria, Speakers Bureau; Bioverativ/Sanofi: Research Funding; Octapharma: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Speakers Bureau; CSL Behring: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.